Alzheimer’s drugs are very valuable for NHS
 Getty images
Getty imagesTwo success Alzheimer’s drugs are considered very expensive, for very little profit, to be introduced on NHS.
Medications are the first to slow down the disease, who can give people extra time to live independently.
The National Institute for Health and Care Excellence (NICE) concluded that they were poor use of taxpayers and said that they could have cut other services from funding.
Preachers say it is a disappointment, but other dementia experts have also supported the decision.
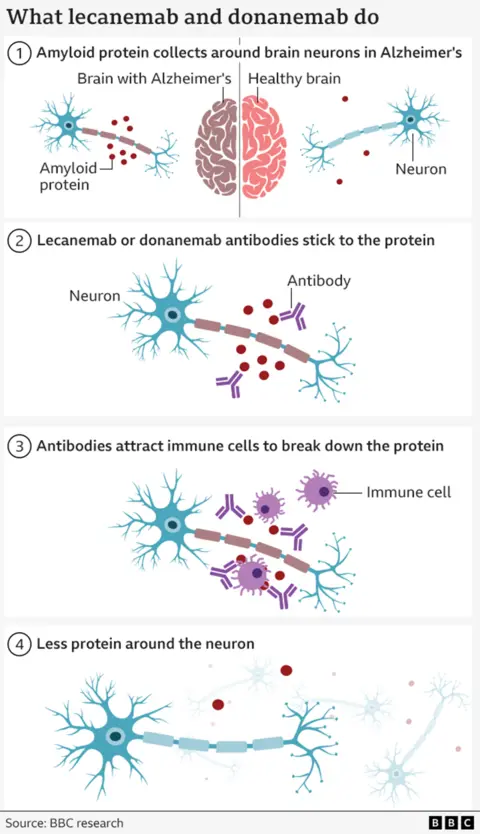
Two drugs, Donanamb and Lekainamb, both help the body clean a ganglogue protein that is formed in the brain of people with Alzheimer’s disease.
Medications do not stop or even prevent the disease, but the strength of the brain is lost more slowly with treatment.
The clinical trials of these drugs were observed as a scientific victory as he showed, for the first time, it was possible to change the course of Alzheimer’s.
But since then a line has developed on the cost of drugs and how meaningful the profit is.
The official price in the US is £ 20,000- £ 25,000 per year. The NHS that pays is confidential.
About 70,000 people were eligible in England with mild dementia, possibly bills in the field of £ 1.5BN per year for drugs alone alone.
NHS resources, including infecting drugs every two to four weeks and repeated brain scan to manage dangerous side effects, will also increase the cost on a large scale.
The benefits of medicines are also debated. They probably delay infections in light to moderate dementia for four to six months. This may mean that there may be more time without the need to be present for daily care, driving, important family programs and socialization.
But Professor Rob Howard of University College London said that the real world benefits were “very younger to be noticeable”. In Lenmab’s tests, patients were better than 0.45 points on a scale of 18 digits from healthy to severe dementia.
Nevertheless, he said that the cost would be close to the cost of a nurse’s salary for each treated patient “.
The decision not to fund medicines is not surprising. The first assessment of last year was concluded that they were not cost effective.
Helen Knight, director of the Medicine Evaluation at NIS, admitted that the latest news would be “disappointing”, but said the benefits were “modest” during the need for “adequate resources”.
“If they are approved, they can displacing other essential treatments and services that provide significant benefits to patients,” she said.
Nees stated that its evaluation had a factor in potential savings in the cost of providing care, but drugs were still considered ineffective.
In England, good decisions on NHS apply, but are usually adopted by Wales and Northern Ireland. Scotland has its own way of approving drugs.
Pharmaceutical companies have concerns about how the reviews were done, otherwise the decision is final on 23 July.
Including both pharmaceutical companies, Christians for Lekenamb and Eli Lily for Dononmab say they will appeal against the decision.
Nick Burgini said to Christian that NHS is “not ready” to deal with Alzheimer’s and deal with flaws. Their drug would have been rejected “even though Christians provided NHS for free”.
Eli Lily, the company behind Dononmab, has already expressed her disappointment.
“If the system cannot provide scientists first to NHS patients, it is broken,” said Chris Stokes, President of Ellie Lily and General Manager of UK and Northern Europe.
Is this a distraction or despair?
The emotion was echoed by both charity Alzheimer’s. Alzheimer’s Society professor Fiona Kargher said “science is flying but the system is failing” and it was “highly disappointing” that drugs were not available on NHS.
Alzheimer’s CEO Hillary Evans-Nuton, Chief Executive Officer of the UK, said that the result was “painful” and patients would remember this and future innovations “because the science is failing, rather because the system is”.
However, others say that Nice has made the correct call. Tom Denning, Professor at Dementia Research at the University of Nottingham, stated that he was “in full support” because the benefits of drugs were “minimal” and there was a “distraction” from real issues in dementia.
He said, “(ie) the uncontrolled challenge of providing people with activities, care and support to dementia and their families who already know that we already know that their mental and physical health are beneficial,” he said.
Professor Aticus Hasworth, Professor of St. George of London University, said: “Nice is just doing his work.”
138 dementia drugs are being tested in 182 trials worldwide beyond Lecanemab and Donenamab.
Director of Center for Discovery Brain Sciences at the University of Edinburgh, Prof. Tara Spire-Jones said: “Hope for safe, more effective treatment on the horizon.”

Get our major newspaper with all the headlines that you need to start the day. Sign up here.



