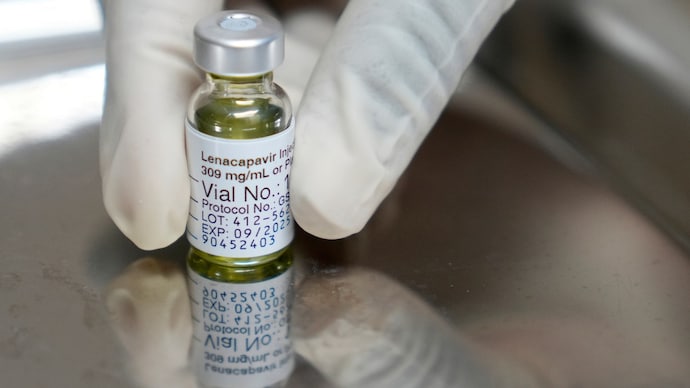
Lenacapavir first becomes FDA-approved HIV preventive medicine: all about it
The US FDA has approved Lenakapvir sold under the brand name Yezatugo as the first preventive treatment against HIV. It is a twice annual injection drug developed for adults and adolescents.
In short
- Lenacapavir is the first twice an annual injection HIV prevention in the US
- Clinical trials showed about 100% effectiveness in preventing HIV infection
- The drug targets several stages of the HIV life cycle for strong safety
In a major success in the fight against HIV, the US Food and Drug Administration (FDA) has approved the Lenkapavir, under the brand name of a long acting injectable drug acting by Gilled Sciences as a preventive treatment for HIV. This has been over two decades.
Lenakpavir for HIV prevention has not been approved by any regulatory authority outside the US. There is currently no cure for HIV or AIDS.
This makes Yeztugo the first and the only HIV pre-exist Profilaxis (Prep) option in the United States that is required only twice a year.
Injectable drug is approved for use in adults and adolescents, which weigh at least 35 kg, which is at risk of obtaining HIV through sexual contact.
Clinical trial data shows over 99.9% of people who receive Yaztugo, remained HIV-negative during the study period, highlighted their strong ability to prevent HIV infection.
Daniel O’De, CEO of Gilid Sciences, said, “This is a historic day in a long battle against HIV for decades.
Sports for HIV Prevention
The first prep medicine made by Gilliad was also approved in 2012. But by 2022, about 1 out of 3 out of 3 people in the US that were eligible for prep, it was actually determined, according to the disease control and prevention center (CDC).
Many people, especially women, continue to face challenges such as stigma, lack of awareness, lack of awareness, and difficulties such as people in the south of the US.
Professor of Medicine at Amori University, Dr. Carlos del Rio said, “Yezatugo can be the transformational option we are waiting for. Twice can help remove major obstacles such as shots and stigma.”
How does Yeztugo works
Yeztugo includes Lenacapavir, a unique antiretroviral that works by blocking HIV (human immunodeficiency virus) in several stages of its life cycle, which is unlike most of the drugs targeting only one.

It is not complete treatment for those who already have HIV, and people should be tested for HIV before starting injections.
The company has also highlighted some drug safety warnings:
- People should be confirmed HIV-negative before starting or continuing Yezto. Taking it inadvertently while being infected with HIV can lead to drug-resistant strains of the virus.
- Common side effects include injection site reactions, headaches and nausea.
- Since the drug remains in the body for 12 months, left doses or improper use can increase the risk of infection and drug resistance.
Yezto is not used by individuals who are HIV positive or whose condition is unknown.
How is it given
Yeztugo is given as a subcutaneous injection every six months, but begins with a loading dose consisting of two injections and oral pills.
If a patient recalls a scheduled injection, temporary oral pills can be taken weekly until the injection resumes.
Clinical test success
The FDA is approved based on two large phases 3 tests, headed by researchers at the Emori University and Gradi Health System.
Objective 1 test: Powered in Sisgender women in sub-Sahara Africa, this test showed zero HIV infection among 2,134 people, who obtained Yeztugo, performed 100% effectiveness compared to daily oral prep (Truvada).
Objective 2 Tests: Different countries include Sisgender men and gender-class people. Of the 2,179 participants, only two HIV infections were recorded in Yezatugo, which showed 99.9% effectiveness.
Both tests found Yejtugo better than daily oral prep and it was generally well tolerated, with no new security concern.
In the light of this success, the colleague reviewed by the journal Science named Lenakpavir 2024, which is 2024 “success of success”.
It is widely accessible in America
To make Yeztugo widely accessible, gillids are working with insurers and health systems to include drugs in insurance coverage.
For individuals unlicensed, the aid program of gillid will provide Yeztugo free from cost, based on eligibility.
Global expansion plans
Gillid has already filed for regulatory approval in Australia, Brazil, Canada, South Africa, and has submitted applications to the European Medicine Agency.
The company is also preparing applications in countries such as Argentina, Mexico and Peru, which rely on FDA approval for their procedures.
So far, Yeztugo is approved in the United States only for HIV prevention. There is no cure for HIV or AIDS, but preventive equipment such as Yeztugo brings the world closer to control epidemic.
With the approval of this FDA, the drug can help those who are struggling with traditional prevention methods, making prevention more manageable.


