WHO prequalifies new HPV single-dose vaccine: know all about it
WHO has prequalified Secolin, a new HPV vaccine developed in China, for single-dose use.
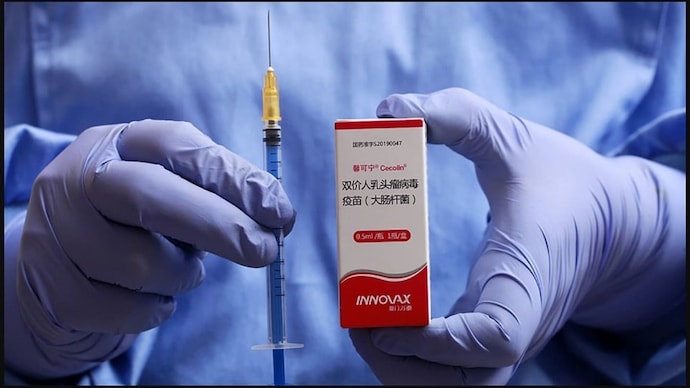
The World Health Organization has prequalified a new human papillomavirus (HPV) vaccine, Secolin, which can be used in a single-dose schedule.
Based on recent data, this is the fourth HPV vaccine to receive such approval that meets WHO’s 2022 guidelines for the use of HPV vaccines in a single dose.
Prequalification is the process by which WHO evaluates vaccines or medicines to ensure that they meet international standards of quality, safety and efficacy. Once a vaccine is pre-qualified, it is considered suitable for distribution in low- and middle-income countries.
Secolin is the first human papillomavirus (HPV) vaccine produced in China that can provide adult women with complete immunity against two strains of the virus. It is manufactured by Xiamen Innovax Biotech Co., Ltd.
Its prequalification means there are more vaccines on the market to help fight cervical cancer, especially in areas facing vaccine shortages, allowing more girls to get protection.
HPV is the main cause of cervical cancer, accounting for more than 95% of the 660,000 global cases each year.
Every two minutes a woman dies of cervical cancer, with 90% of these deaths occurring in low- and middle-income countries, particularly in Africa.
HPV vaccines are critical in preventing these deaths, yet their availability has been hampered by global supply shortages since 2018. Recent production issues have worsened this shortage, particularly affecting girls in Africa and Asia.
“We have the potential to eliminate cervical cancer. By adding another option for one-dose HPV vaccination, we are one step closer to consigning cervical cancer to history,” said Dr. Tedros Adhanom Ghebreyesus, WHO Director-General.
WHO’s global strategy aims to fully vaccinate 90% of girls against HPV by the age of 15 years.
Dr. Kate O’Brien, director of WHO’s immunization department, said the introduction of single-dose vaccines would give countries more options and help them reach more girls despite ongoing supply challenges.
Secolin is one of several vaccines that were initially approved for a two-dose schedule, but can now be used as a single dose. This type of “off-label” use is based on data that shows its effectiveness, even though manufacturers have not yet updated their labels.
As of September 2024, 57 countries have adopted the single-dose schedule, up from 37 in 2023. This change would allow an additional 6 million girls to receive the vaccine in 2023 alone.
Additionally, the fifth HPV vaccine, Valrinvax, was prequalified by WHO in August 2024.
This vaccine has been approved for a two-dose schedule, but further research will determine whether it can be used in a single-dose schedule in the future.


